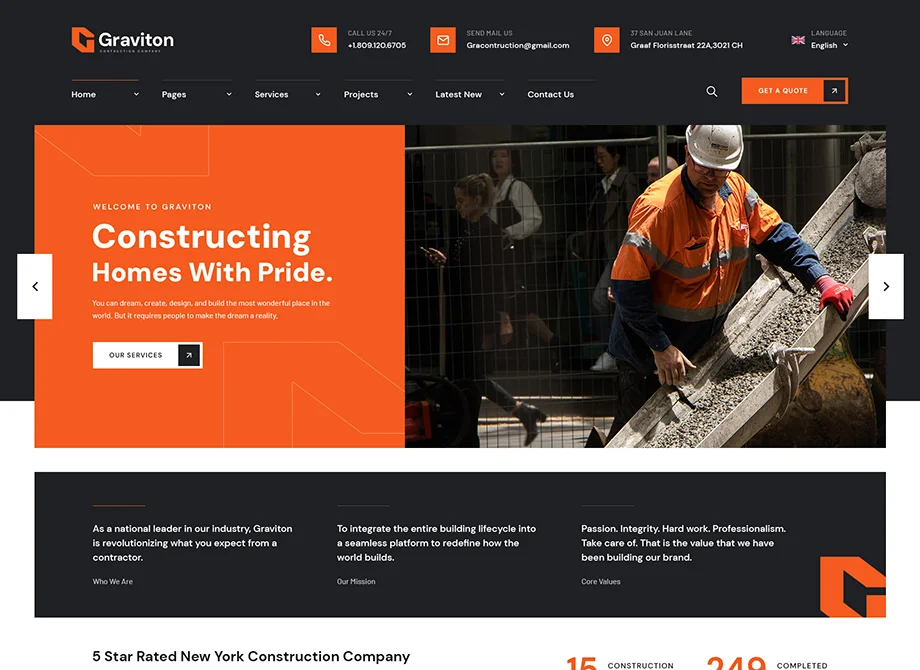
Constructing Homes With Pride
Our diverse portfolio represents decades of construction experience backed by a passion for quality, outstanding client service and the latest industry.


We build your
homes with trust.
Our diverse portfolio represents decades of construction experience backed by a passion for quality, outstanding client service and the latest industry.
As a national leader in our industry, Graviton is revolutionizing what you expect from a contractor.
Who We Are
To integrate the entire building lifecycle into a seamless platform to redefine how the world builds.
Our Mission
Passion. Integrity. Hard work. Professionalism. Take care of. That is the value that we have been building our brand.
Core Values

5 Star Rated New York Construction Company
Quality construction. Inspired design. Unparalleled experience. Exemplary service.
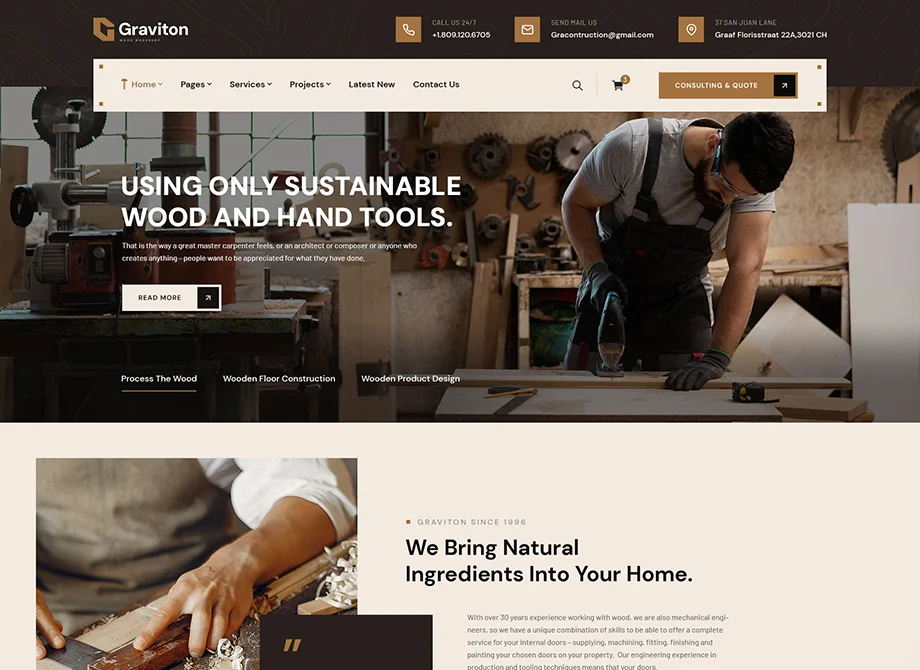
Building Your Visions. Creating Reality.
We build multi-family and affordable housing communities, industrial facilities, public and private healthcare facilities, fitness centers and office buildings. We improve the supply chain management process, increase operational efficiencies and build environments that foster creativity. Our commitment to sustainable construction helps improve the communities in which we build.
The structures we create provide our clients with more than just buildings – we deliver environments that help them achieve their goals.

Explore Our Services
Graviton construction is dedicated to being a partner for all of your construction needs.

Building Construction
-
Build to Suit
-
Build Operate Transfer
-
Public-Private Partnership
Architecture Design
-
Schematic Design
-
Bidding and Negotiation
-
Electronic Project Archive
Building Renovation
-
Create a Home Office
-
SKitchen & Bathroom Remodels
-
Whole Home Remodels & Additions
Flooring & Roofing
-
Build to Suit
-
Build Operate Transfer
-
Public-Private Partnership
General Contracting
-
Schematic Design
-
Bidding and Negotiation
-
Electronic Project Archive
Repair & Expand
-
Create a Home Office
-
SKitchen & Bathroom Remodels
-
Whole Home Remodels & Additions
Why Choose Us?
Developed in close collaboration with our partners and clients, combines industry knowledge, decades of experience, ingenuity and adaptability to deliver excellence to our clients.

Our construction company is the perfect choice for your dream home. Contact us now today!

Explore Large-Scale Project
Bring to the table win-win survival strategies to ensure proactive domination. At the end of the day, going forward, a new normal that has evolved from generation .
Energy Sciences Building
Electrical Equipment Safety
School Electrical Infrastructure
Household Electricity
Orange Architecture Building
The Ivey School of Business
Cascades Casino Delta
General Constructions
Institutional Design
Parametric Design
Two St. Thomas
Atrio Torre Norte


We Have Become The Best In What We Do
Do you offer a free, no obligation quotation?
Is there a waiting list for desired work to be started?
How are payments handled and dealt with?

Meet Our Leadership
We are driven to improve the lives of our clients, our employees, and our community through our commitment to leadership, excellence in craft, and attention to detail.
Penelopa Miller
Stephan W.Lars
Steven Berkoff
Albert Flores
Penelopa Miller
What Your Client Say?
Mr. R. Ramanujam
Andrew J Cawthray
Stuart Clark
Get In Touch With Us
If you need to repair or replace your Plumbing system, call today and talk to one of our Plumbing specialists.
Call support center 24/7
Graaf Florisstraat 22A,3021 CH
Gracontruction@gmail.com
Articles & Blog Posts
Bring to the table win-win survival strategies to ensure proactive domination. At the end of the day, going forward, a new normal that has evolved from generation .